A perfectly ordinary spoon. It looks and feels like aluminum, just a bit heavier…unless you hold it too long. But the person who handed it to you urges you to immediately mix your coffee with it. In a matter of seconds, you have in your hand only the handle, which soon begins to melt and also drip into the coffee cup! What is this?
Gallium, which is chemically very similar to Aluminum, is nearly twice as dense, but still much less dense than stainless steel or silver. A spoon made of it feels light. Its melting point is 85.6°F (29.8°C), and your hand is several degrees warmer than that (unless you've just been outside throwing snowballs with bare hands). If you had held the spoon in your hand more than a few seconds, it would have begun to melt. Gallium and Mercury are the only two metals that you can touch when they are molten without getting a serious burn. There are hundreds of YouTube videos showing gallium spoons, and other objects, melting in warm water or into the hand of someone. It is not toxic, while mercury is very toxic.
Stories about gallium and other elements—all of them, in fact—fill the pages of The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World From the Periodic Table of the Elements, by Sam Kean. "The Periodic Table!" you say, perhaps with a shudder. Shades of Junior-class Chemistry in High School rise up to haunt you.
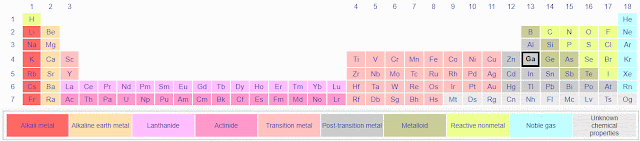
The Periodic Table of the Elements isn't (only) an instrument of teen torture. It is very useful. Just to lay some groundwork, and to get it out of the way, here is a simple version as seen in the Gallium page of Wikipedia:
The version you most likely remember (or try to forget) probably had 18 columns, not 32, with a separate pair of lines below with elements running from La to Lu and Ac to Lr (or maybe only as far as Cm or Bk with the rest of the boxes blank). This table incorporates all the elements into a single table and adds the convenience of color coding of elements with similar, or related, chemical properties. Gallium (Ga) is highlighted, right below Aluminum (Al).
The chemical similarities between certain elements led to the development of the first Periodic Table by Dmitri Mendeleev in 1869. He was not the first to notice the similarities, but he was the first to use the "periods" (the columns in the table and their repeating nature) to predict the placement and chemical properties of several new elements. As these elements were discovered, one after another, the table was established, as was Mendeleev's fame.
Early chapters of The Disappearing Spoon outline Mendeleev's discovery and that of the first handful of elements, including the ones that confirmed that his table was trustworthy. Other chapters group the elements by the kinds of stories that swirl around them (or did when they were newly known). For example, Chapter 9, "Poisoner's Corridor: 'ouch-ouch'", begins with the sad story of cadmium (Cd) in a Japanese mine. Waste material rich in Cd and zinc (Zn) was a byproduct of the precious metals that the Shogun desired. Later, when Zn was found useful, the waste tailings were re-mined, of course without any protective measures. You'll see Cd in the table above, just below Zn, which is next to Ga. Being chemically similar, Cd is found with Zn, but it is not totally identical so acid roasting can separate the two metals. Cd-rich waste, now in water-soluble form, was cast away and got into the streams. People downstream who drank the water got "Itai-itai!" disease. "Itai" is Japanese for "Ouch!" Cd weakens the bones and later causes the kidneys to fail. Early death was nearly universal, either because of infection from compound fractures, or from kidney disease. Thallium (Tl) and Bismuth (Bi) have their own stories to tell, of chemical "improprieties". Strangely, in the right compound, Bi is not toxic, and is the basis for Pepto-Bismol! You can drink it to help an upset stomach. Tl, on the other hand, is horrific! Read about it.
Two other elements, Thorium (Th) and Americium (Am) poison in a different way. They are radioactive. They bracket the range of the more common radioactive elements. The most stable isotope of Thorium, Th-232, has a half-life of 14 billion years, nearly three times as long as Uranium-238. As metals, Th and U are safe to handle for short times (I have done so). Am-241 is much more dangerous than radium (Ra). Its half-life is 432 years, while that of Ra-226 is 1,600 years. However, while radium decays to radon (Rn), the radioactive gas that we try to keep out of our basements, americium decays to other metallic elements, which stay put. Thus it can be used in smoke detectors, so you probably have a few micrograms of Am-241 in your house!
Chapter after chapter, the author tells us story after story of how elements were discovered, and/or how they are used, and curious facts about them. The phrase "Mad as a Hatter" (and the Mad Hatter of Alice in Wonderland) indicate the gradual insanity caused by using mercury (Hg, for "hydrargium" or "liquid silver") to process felt for hat-making. And some new elements have been tried as love potions, with universal lack of success.
So, maybe you hated high school chemistry, or maybe, like me, you loved it. Regardless, the stories behind the elements, and their arrangement into the Periodic Table, are enjoyable. The book is well worth a read.
I have to close with a quibble about near-homonyms and their misuse. Referring to metals such as Cd being dissolved into ground water, the author uses both "leech" and "leach", where only "leach" is proper. The other word refers to a parasitic animal, or your cousin who is always borrowing from you. Then, he describes the damage and illness caused by some elements as being "ravished". The right word is "ravaged". To ravish is to rape, though the word has other uses, but it is definitely not a synonym for ravage. And finally, describing physical characteristics of some animals, "waddles" appears. The author meant "wattles", the folds of skin on the neck of a turkey or an elderly person or dog. "Waddle" is a verb, not (or very rarely) a noun, and describes walking with a distinct swaying motion.
Well, let's forgive Sam Kean for such minor crimes. His writing is enjoyable and the stories he has unearthed are fascinating. I bought this e-book as part of a three book set, so it won't be long until I review the third (I already reviewed The Tale of the Dueling Neurosurgeons).



No comments:
Post a Comment